The social mindset is the result of an active link between brainstem responses and episodic memories.
It is the product of a human-expanded brain network consisting of two main regions of the brain:
The mechanism of the social mindset is fully described in the rest of this page, but it can be summarised in short as the following:
- Signals of brainstem responses (emotions such as laughing/crying, sexual arousal, yawning, etc.) received by the anterior insular cortex are attached to episodic memories as they are formed and stored in the medial prefrontal cortex.
- Episodic memories with these attached brainstem-response ‘signatures’ directly trigger the same brainstem response later on when something similar to them is witnessed or when they are thought of.
- Witnessed phenomena that are similar to existing episodic memories attached to brainstem-response signatures become attached as new episodic-memory nodes of the social mindset.
Before the social mindset, episodic memories consisted only of sensory input, and they merely regulated behaviour around innate survival mechanisms (such as satiety or pain perception), which themselves would trigger the brainstem responses.
The anterior insular cortex and anterior cingulate cortex are right-lateralised,[1] and their right-hemisphere regions are around 13% larger than the left.[2] The right-hemisphere regions respond more to sympathetic-nervous-system signals (‘fight-or-flight’ signals), while the left-hemisphere regions respond more to parasympathetic signals (‘rest-and-digest’ signals).[3]
A 2009 article summarised that the anterior insular cortex and anterior cingulate cortex are activated during feelings such as ‘maternal and romantic love, anger, fear, sadness, happiness, sexual arousal, disgust, aversion, unfairness, inequity, indignation, uncertainty, disbelief, social exclusion, trust, empathy, sculptural beauty, a ‘state of union with God’, and a hallucinogenic state (induced by ayahuasca).’[3]
In 1991[4] (elaborated in 1996[5]), before the significant details of this network were known, António Damásio had proposed the ‘somatic marker hypothesis’, involving the attaching of interoceptive emotional ‘markers’ to memories, to explain emotional decision-making in gambling.
Anterior insular cortex
The insular cortex (or insula) is a region of the cerebral cortex beneath the frontal and temporal lobes. It is closely linked to the brainstem and nerves via the corona radiata and is involved in regulating an agglomeration of bodily sensations, including those of touch, pain, temperature, taste, sound and heartbeat.[6] These signals are received in the posterior insula, which also receives signals from varied parts of the cortex and limbic regions.[7]
The human anterior insula is a specialised region of the insula that integrates brainstem signals and forwards them to other regions of the brain.[8][9] The anterior insula is agranular, containing fewer layers than the six of most of the rest of the cerebral cortex.[10]
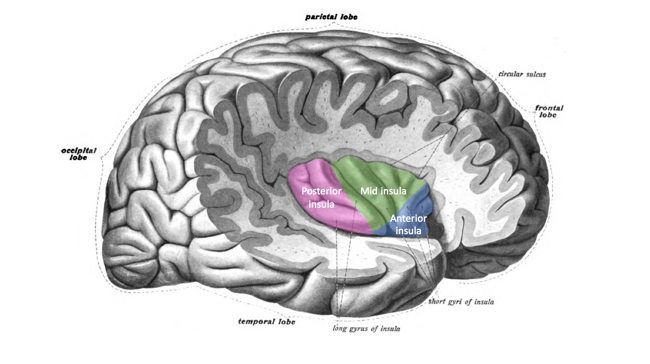
Source: [11]. Licence: CC-BY-SA-4.0.
Anterior cingulate cortex and medial prefrontal cortex
The anterior cingulate cortex (ACC) is a region of the brain in (or inferior to) the medial prefrontal cortex (mPFC). The ACC, especially the dorsal ACC,[12] can generally be described in terms of the functions of the surrounding mPFC, to which it serves similar roles.[13]
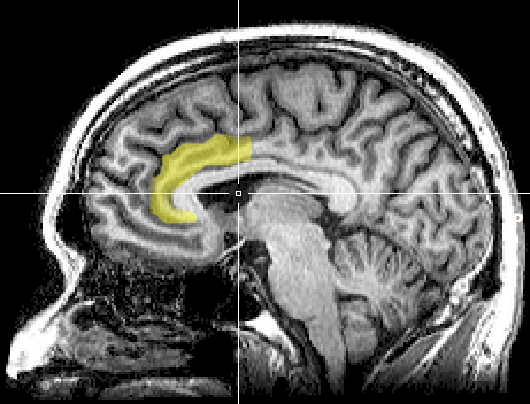
Source: [14]. Licence: CC0-1.0.
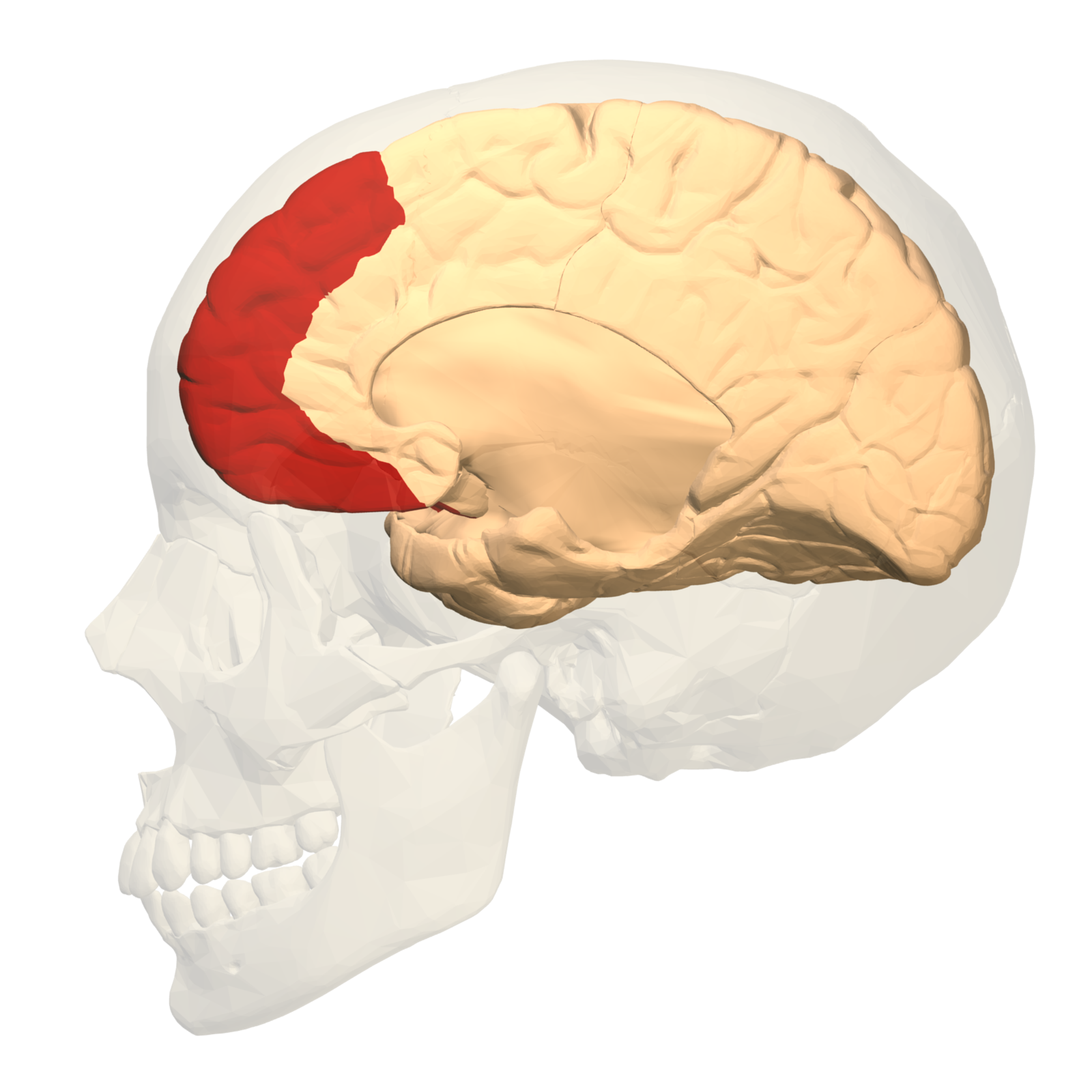
Source: [15]. Licence: CC-BY-SA-2.1 JP.
The mPFC is the centre for the long-term storage of episodic memories. Episodic memories are memories of events that occurred over time (as opposed to semantic memory, timeless memory of facts) and consist of an agglomeration of all sensory inputs.
Episodic memories are stored in the mPFC according to their commonalities and are used to predict consequences, characterise phenomena, form expectations, decide on an action, inhibit an action or express a consciously learnt anxiety of a situation (rather than an imprinted anxiety from early childhood).[16][17]
Like the mPFC, the ACC is involved in performing evaluations and corrections of actions[12] and consolidating learnt anxieties.[13]
The dorsomedial prefrontal cortex is involved in the memory storage and association, while the ventromedial prefrontal cortex (vmPFC) links the region to the reward/aversion centres.[16] The vmPFC’s reward and aversion roles are facilitated by its connections to the striatum of the basal ganglia and the basal nucleus of the amygdala.[16]
Similarly to the vmPFC, the ventral ACC is more linked to limbic areas and is more active when consolidating a reward in association with the ACC’s activity[12] as well as expressing learnt anxieties via its projections to the amygdala.[18]
Encoding in the mPFC for the long-term depends on its connections from the hippocampal region, which are theorised to be the initial source of the short-term memories and visuospatial contexts to the mPFC, as the hippocampus is with other regions of the brain.[17]
Von Economo neurons and connections
The fronto-insular cortex, comprising the anterior insula and adjacent ventrolateral prefrontal cortex,[19] along with the ACC and the vmPFC, are some of the only regions of the human brain known to contain von Economo neurons.
Von Economo neurons (VENs) are large, long, thin, spindle-shaped cortical neurons that are bipolar (having a single dendrite and a single axon), in contrast to the typical pyramidal neurons of the cerebral cortex, which have multiple dendrites and one axon.
VENs have only been found in a select group of mammals with relatively developed social behaviours. They have been found in great apes (humans, bonobos, chimpanzees, gorillas and orangutans), the macaque (in the anterior insula),[20] a number of cetaceans (dolphins, whales, etc.)[21][22] and elephants.[23] In these species, they have been theorised to overcome transmission delay between their respective brain regions, since larger axons have faster transmission.[24][25]
Humans have multiple times more VENs than other apes, whose total numbers generally decrease with phylogenetic distance from humans.[26] The human insula also has considerably more variability among individuals than that of other primates.[27] In macaques, the pregenual ACC (the front part of the ACC) is involved in spontaneous social interaction, shared experience and social reward.[28] Rats and lizards lack an analogous anterior insular cortex.[3]
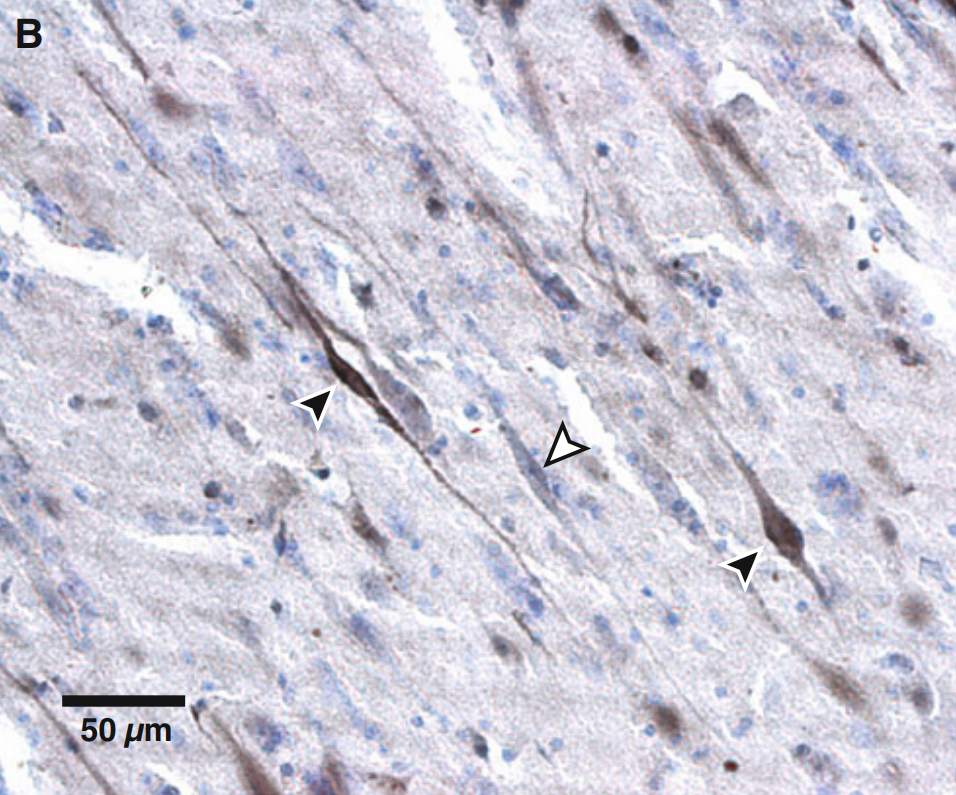
Source: [2]. Licence: CC-BY-NC-2.0.
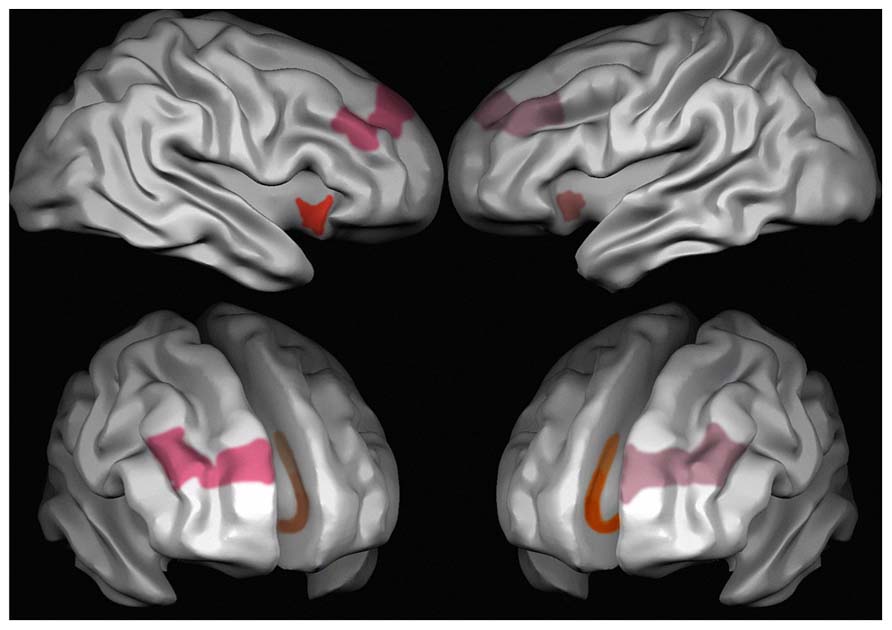
Red: anterior insula; pink: Brodmann area 9 of the prefrontal cortex;[29] orange: anterior cingulate cortex.
Source: [30]. Licence: CC-BY-3.0.
Source: [32]. Licence: CC-BY-3.0.
Studies have theorised some form of transmission from the anterior insula to the ACC,[3][6][25] however studies have characterised the VENs as neurons that project from the cortex to the brainstem and limbic regions.[7][22][33][34][35][36] A 2018 study found only sparse direct connections between the anterior insula and ACC (mostly between the non-VEN areas).[37]
Rather, in line with the mechanism described below and the features, the anterior insula would forward brainstem-response signals as episodic memories are being formed, which would be to regions (likely in the mPFC) other than the ventral ACC.
Likewise, the ventral ACC would most likely serve as the trigger for brainstem responses saved from previous episodic memories and, as such, would be a point of output receiving input from the rest of the mPFC.
The anterior insula and ACC are almost always activated together,[9] but the activation of the anterior insula precedes that of the ACC.[19][25]
In the triple-network model
The network can be illustrated in perspective by the triple-network model by Vinod Menon (2011),[38] which categorises groups of brain regions that share functional connectivity into three main networks:
- The frontoparietal (or central executive) network (FPN) primarily comprises the dorsolateral prefrontal cortex and posterior parietal cortex.
- The salience network (SN) primarily comprises the anterior insular cortex and anterior cingulate cortex.
- The default mode network (DMN) primarily comprises the medial prefrontal cortex, posterior cingulate cortex and posterior parietal cortex.
The FPN handles goal-oriented tasks involving sustained attention and working memory, while the DMN is active at rest or during episodic-memory retrieval (such as during daydreaming). The SN is activated by incoming attention-shifting sensory stimuli, which especially disengages the DMN.[38][39]

ACC: anterior cingulate cortex; DLPFC: dorsolateral prefrontal cortex; INS: insular cortex; mPFC: medial prefrontal cortex; PCC: posterior cingulate cortex; PPC: posterior parietal cortex.
Source: [40]. Licence: CC-BY-3.0.
The DMN and SN have repeatedly been found to be associated with empathy, understanding/imagining fiction, self-perception and other features of the social mindset.[41][42][43]
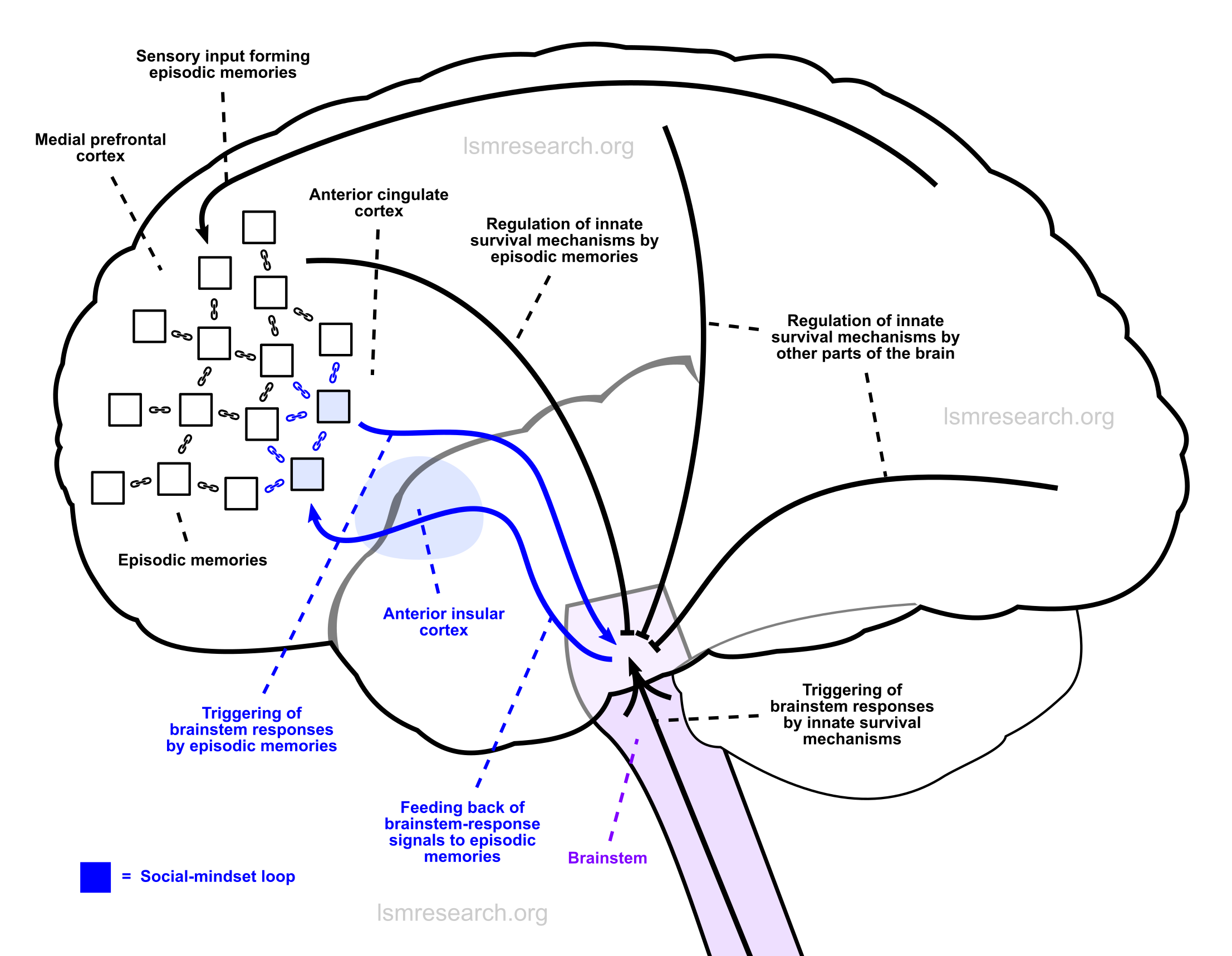
Mechanism
I now theorise in detail the mechanism by which the connection between the anterior insula, mPFC and ACC brings about the social mindset, in line with its features.
Outside the social mindset, episodic memories consist of a combination of anything that was seen, heard, touched, smelt, tasted, etc. at a certain point in time.[44]
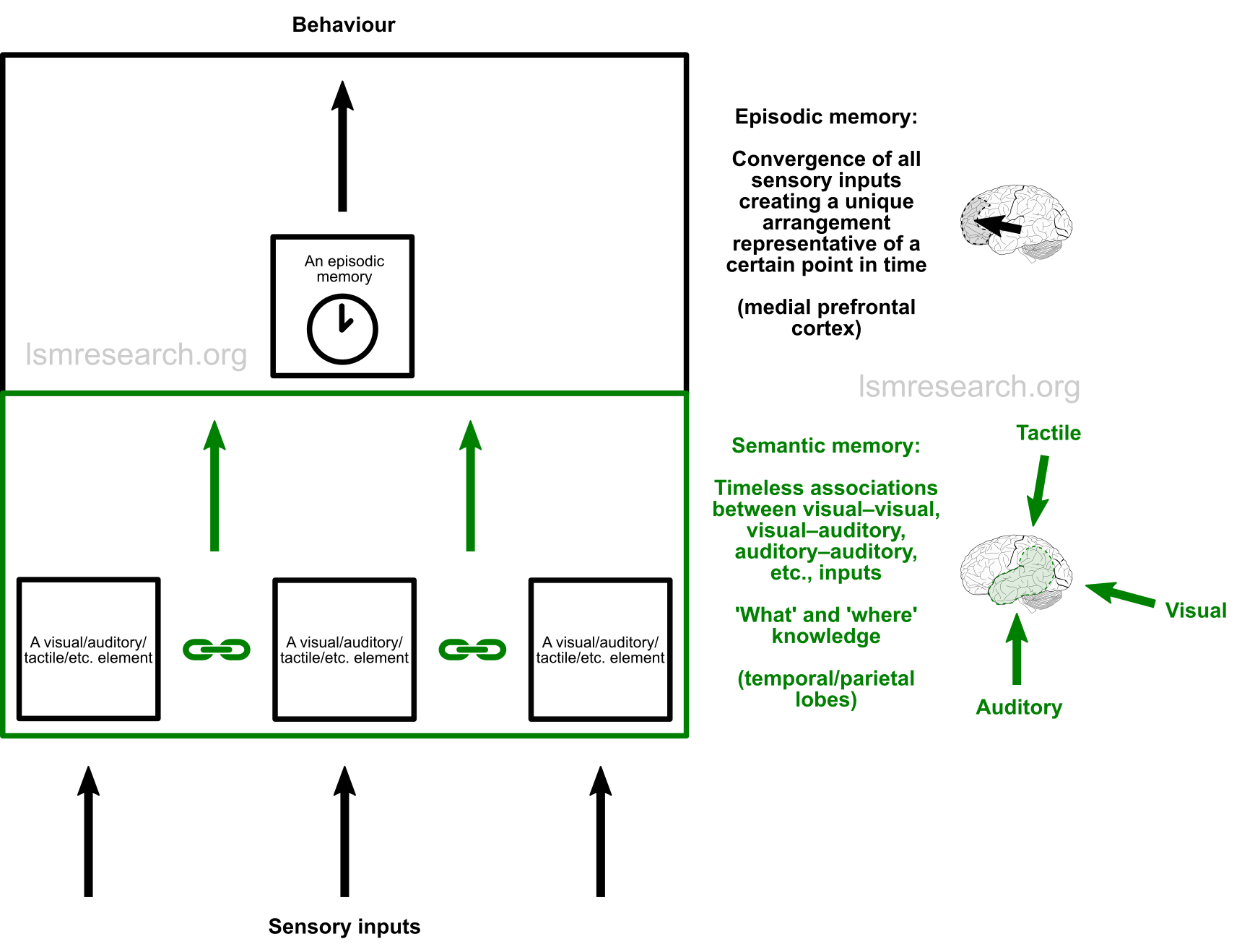
The episodic memories are stored according to commonalities to make predictions and characterise phenomena to guide behaviour towards innate survival goals, such as satiety, avoidance of harm/pain, etc. These innate survival mechanisms elicit the brainstem responses, such as pain leading to stress or anxiety, while the episodic memories merely consist of the sensory input.
However, in the social mindset, there is an active link between the brainstem responses and episodic memories themselves via the anterior insula and ACC.
This leads to two main things:
- a) It allows the signals of the brainstem responses to be attached to the episodic memories as if they were themselves episodic memories.
- b) It allows the episodic memories themselves to trigger the brainstem response rather than an innate survival mechanism.
This completes a loop that otherwise does not exist in the brain.
Since episodic memories are used to make predictions and characterise phenomena, this means that brainstem responses (internal emotions, rather than external input) now form part of what is used to characterise phenomena.
Diagrams
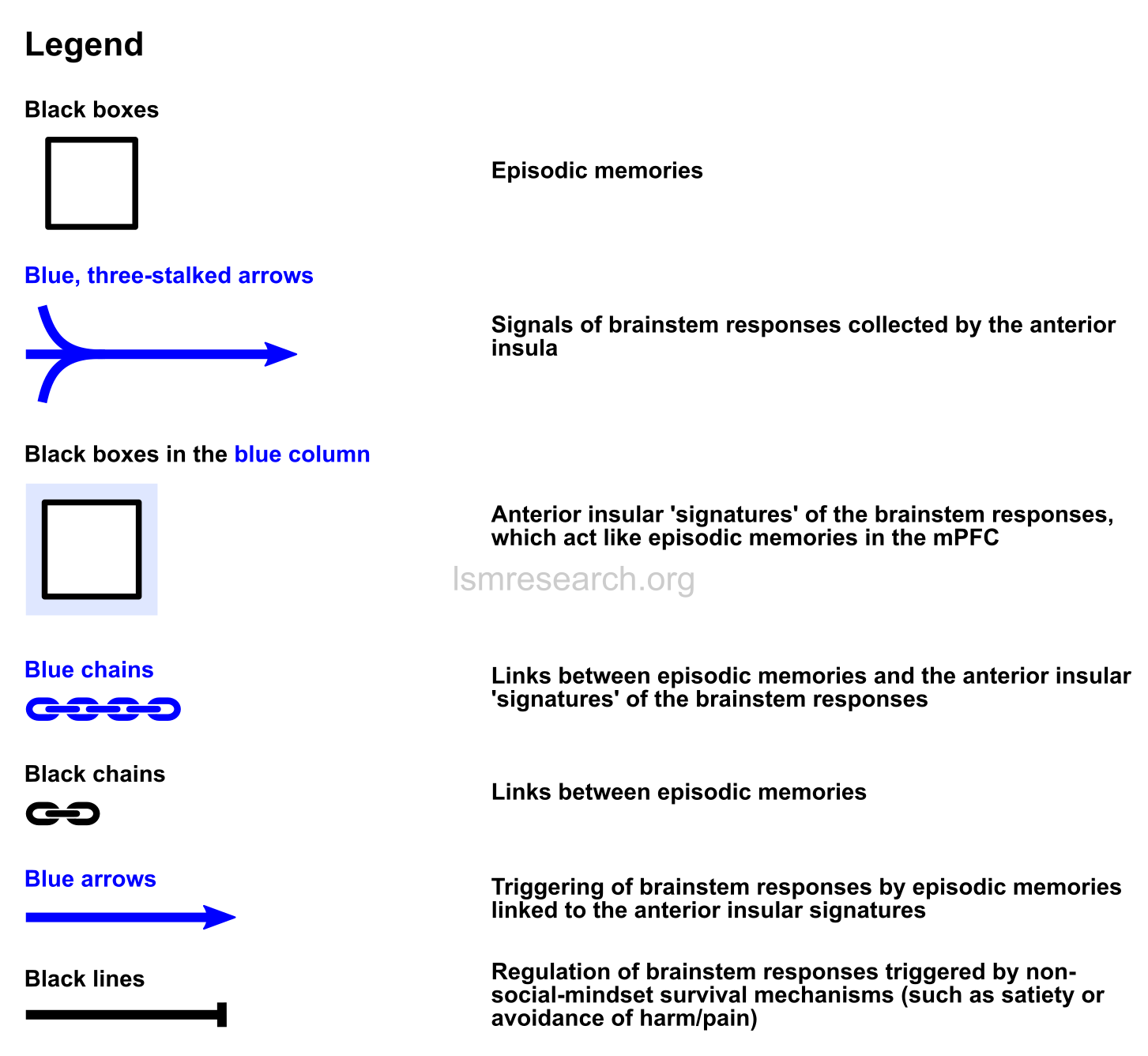
In the following diagrams, the purple column consists of autonomic brainstem responses, such as smiling/laughing, sadness/crying, sexual arousal, yawning, etc.
The blue column consists of the connection between the anterior insula, which collects (blue, three-stalked arrows) the signals of these brainstem responses, and the anterior cingulate cortex, which has connections to episodic memories (black boxes) in the medial prefrontal cortex (the black and white column).
The signals of the brainstem responses are fed back through the anterior insula and attached (blue chain) as active ‘signatures’ (black boxes in the blue column) to episodic memories as they are formed.
This leads to:
- a) the brainstem responses being triggered (blue arrows) when new phenomena are observed that resemble (black chains) the episodic memories;
- b) the brainstem responses characterising the phenomena as if they were themselves episodic memories;
- c) the new phenomena being added as episodic-memory reference nodes for future triggers of and objects of characterisation by the brainstem response.
The red box is the link to the reward and aversion centres of the ventral striatum and amygdala.
All text, chains and arrows in blue comprise the social mindset.
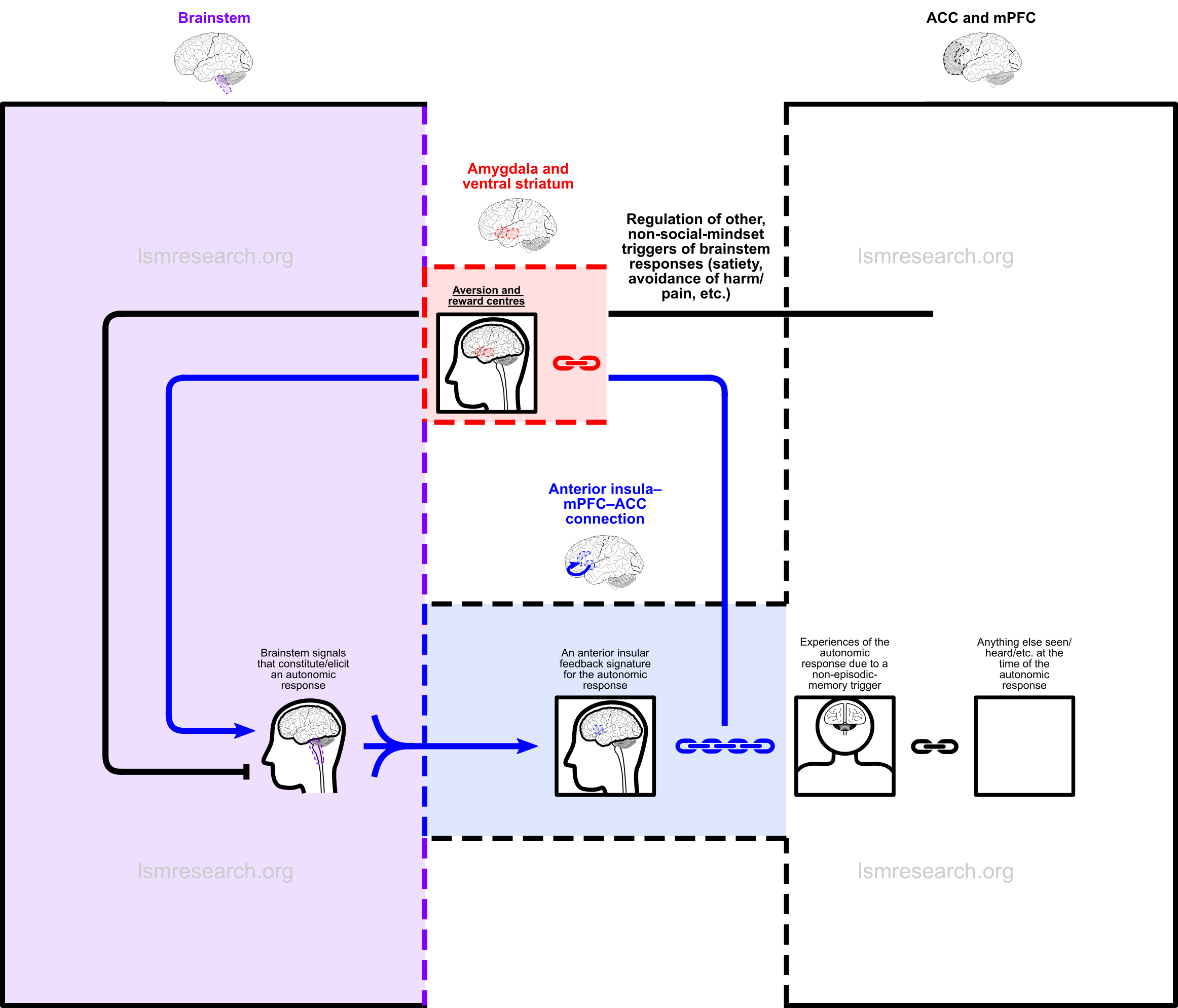
The above diagram shows that the feeding back and attaching of brainstem-response signals takes place constantly as episodic memories are formed. Importantly, it also shows that episodic memories that are attached to this response can consist of anything.
However, what will be clear is that by far the most common observation to correlate with a brainstem response is the behaviours of the self that are able to be witnessed/heard/etc. by the self during the episode, such as, with the crying response, the sounds made when crying, the feel of tears down the face and actions done, such as running to one’s mother. Due to repeated exposure, these will therefore accumulate by far the strongest attachments.
This leads to the following diagram.
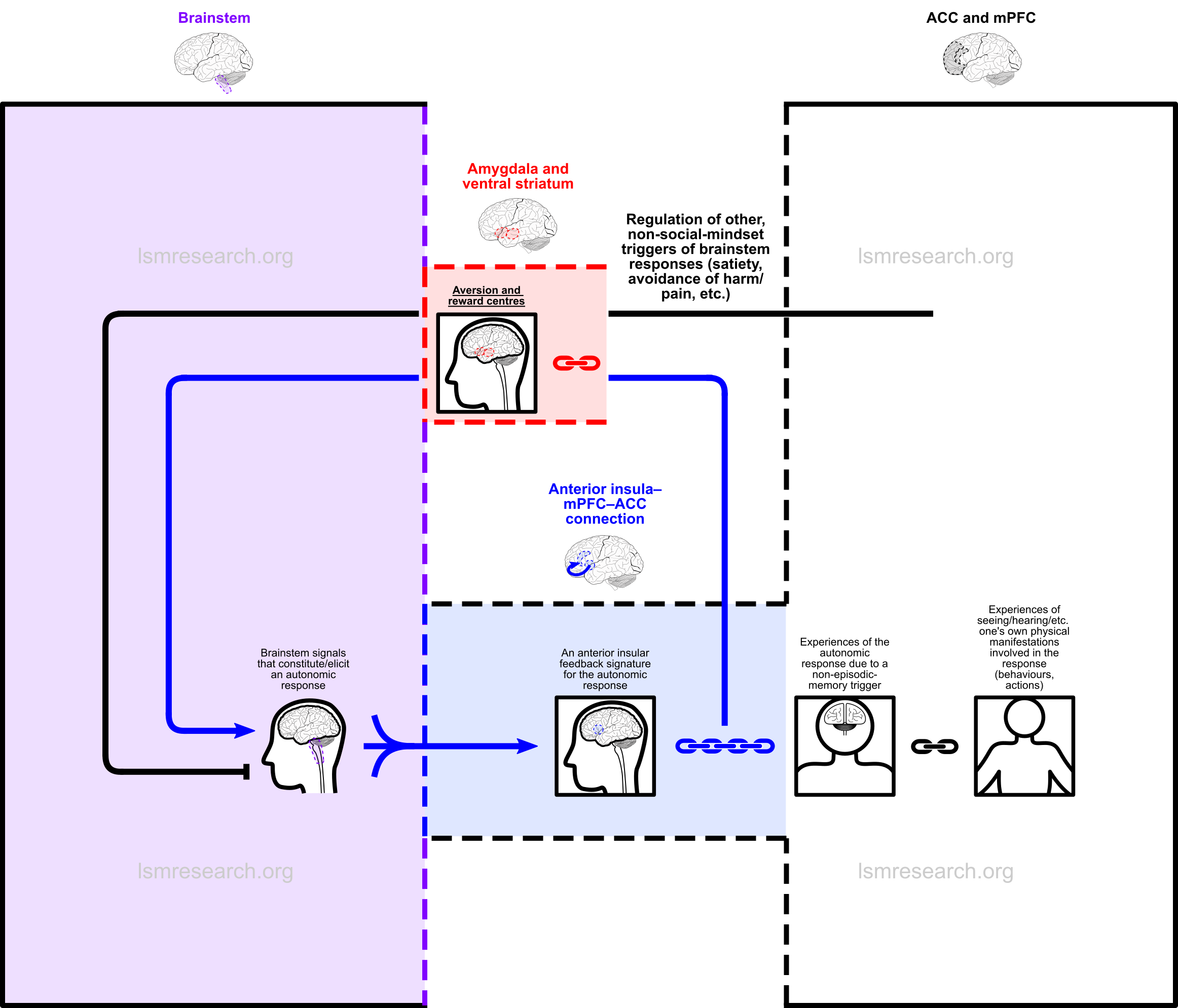
Later on, one will witness another human exhibiting behaviours that strongly resemble those one witnessed during the episodes of the autonomic response (to carry on with the previous example, crying). Once again, what one witnessed during the episodes of the autonomic response did not have to be one’s own behaviours, but due to repeated exposure, these serve as by far the strongest and most reinforced attachment.
The behaviours will be witnessed and compared with what is already stored in the mPFC in order to understand/characterise it, and a match will be made to the memories of one’s own behaviours witnessed during crying episodes.
Once again, in the person with the social mindset, this will have three effects:
- a) the witnessed behaviours will trigger the autonomic crying response in the self;
- b) the autonomic crying response will be used to characterise the witnessed behaviour as if it were itself an episodic memory;
- c) anything new that is currently being witnessed will be attached as a new episodic-memory reference node for future phenomena to trigger and be characterised by the autonomic crying response.
This is shown in the following diagram (for any autonomic brainstem response).
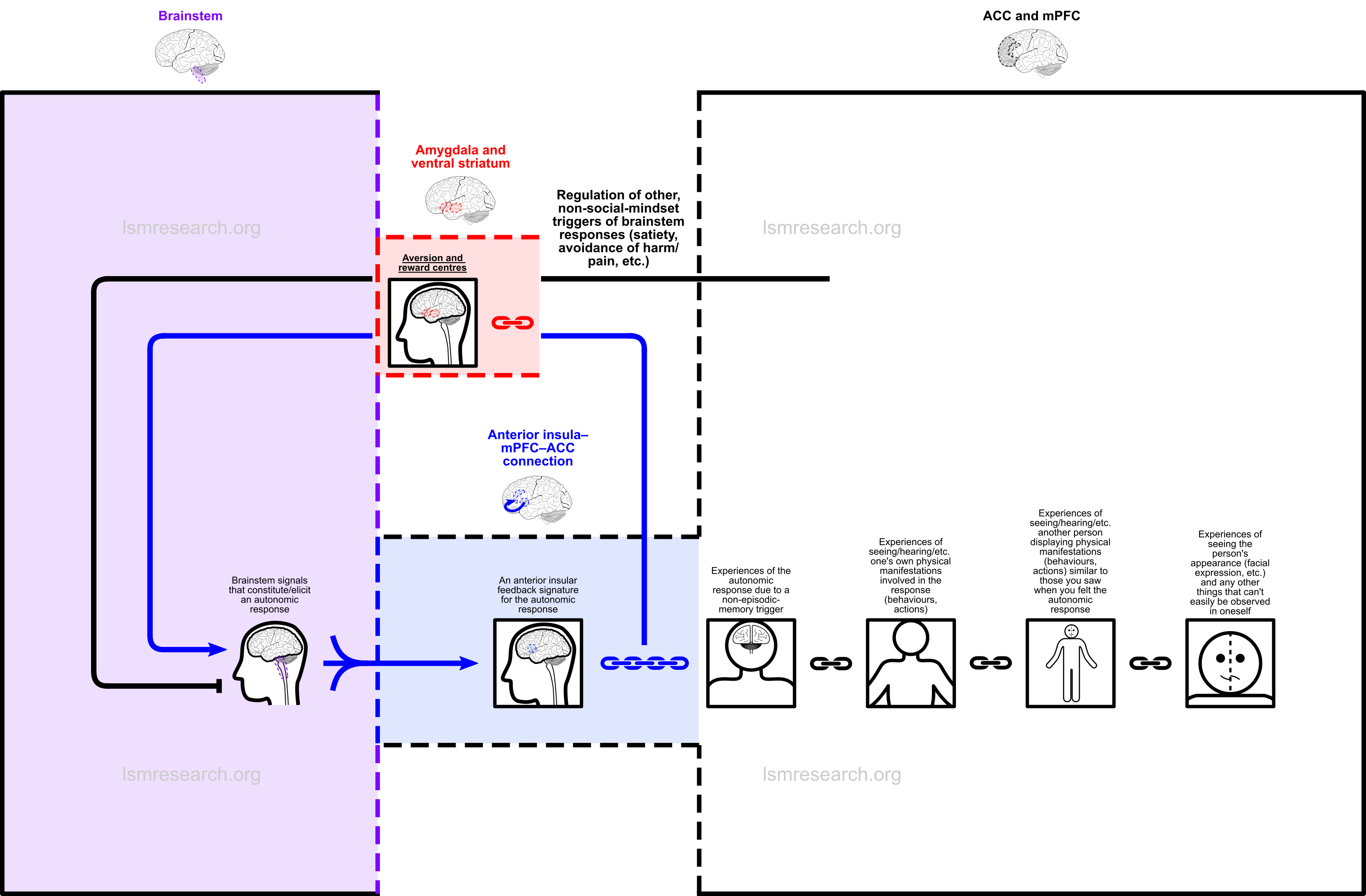
A fourth node is attached during this third-party observation episode: it is every feature of the other person that could not be witnessed in oneself (without the aid of a mirror), most notably the person’s facial expression.
These episodic-memory nodes complete the knowledge of what one’s emotional expressions can look like in others. In those with the social mindset, they set the stage for any witnessed third-party emotional expression to trigger the emotion in the self and have anything new being witnessed at the time be added as a new triggering node and a phenomenon to be characterised by the associated brainstem response. This gives rise to most of the features of the social mindset, including empathy.
However, since one’s behaviours are not the only observation that can serve as a reference node, there are a few other paths the chain can take, which are discussed on Features of the social mindset.
When something similar to a brainstem-response-linked episodic memory is witnessed, it becomes added as a new episodic-memory node of the social mindset, and these build progressively in a tree-like fashion.
With the social-mindset loop, the brainstem forms an integral part of the reasoning, prediction and expectation centre of the brain (the medial prefrontal cortex), meaning that one’s internal emotions (rather than just external sensory input) now necessarily and involuntarily form part of what is used to characterise phenomena that resemble one’s social-mindset-linked episodic memories.
References
- ^ Zhang, Yaodan; Suo, Xinjun; Ding, Hao; Liang, Meng; Yu, Chunshui; Qin, Wen (2019). "Structural connectivity profile supports laterality of the salience network". Human Brain Mapping. 40 (18): 5242–5255. doi:10.1002/hbm.24769. ISSN 1097-0193.
- ^ a b Allman, John M.; Tetreault, Nicole A.; Hakeem, Atiya Y.; Manaye, Kebreten F.; Semendeferi, Katerina; Erwin, Joseph M.; Park, Soyoung; Goubert, Virginie; Hof, Patrick R (2011-4). "The von Economo neurons in fronto-insular and anterior cingulate cortex". Annals of the New York Academy of Sciences. 1225: 59–71. doi:10.1111/j.1749-6632.2011.06011.x. ISSN 0077-8923. PMC 3140770. PMID 21534993.
- ^ a b c d (Bud) Craig, A. D (2009-01). "How do you feel — now? The anterior insula and human awareness". Nature Reviews Neuroscience. 10 (1): 59–70. doi:10.1038/nrn2555. ISSN 1471-0048. (Archive version from 10 January 2013.)
- ^ Damasio, A. R.; Tranel, D.; Damasio, H. C (1991). "Somatic markers and the guidance of behavior: Theory and preliminary testing". Frontal lobe function and dysfunction. 217–229.
- ^ Damasio, Antonio R.; Everitt, Barry John; Bishop, Dorothy; Roberts, A. C.; Robbins, Trevor William; Weiskrantz, Lawrence (1996-10-29). "The somatic marker hypothesis and the possible functions of the prefrontal cortex". Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 351 (1346): 1413–1420. doi:10.1098/rstb.1996.0125.
- ^ a b Uddin, Lucina Q.; Nomi, Jason S.; Hebert-Seropian, Benjamin; Ghaziri, Jimmy; Boucher, Olivier (2017-7). "Structure and function of the human insula". Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 34 (4): 300–306. doi:10.1097/WNP.0000000000000377. ISSN 0736-0258. PMC 6032992. PMID 28644199.
- ^ a b Evrard, Henry C (2019). "The Organization of the Primate Insular Cortex". Frontiers in Neuroanatomy. 13. doi:10.3389/fnana.2019.00043. ISSN 1662-5129.
- ^ a b Cauda, Franco; Geminiani, Giuliano Carlo; Vercelli, Alessandro (2014). "Evolutionary appearance of von Economo’s neurons in the mammalian cerebral cortex". Frontiers in Human Neuroscience. 8. doi:10.3389/fnhum.2014.00104. ISSN 1662-5161.
- ^ a b Craig, A. D (2002-08). "How do you feel? Interoception: the sense of the physiological condition of the body". Nature Reviews Neuroscience. 3 (8): 655–666. doi:10.1038/nrn894. ISSN 1471-0048.
- ^ Chikama, Masanori; McFarland, Nikolaus R.; Amaral, David G.; Haber, Suzanne N (1997-12-15). "Insular Cortical Projections to Functional Regions of the Striatum Correlate with Cortical Cytoarchitectonic Organization in the Primate". Journal of Neuroscience. 17 (24): 9686–9705. doi:10.1523/JNEUROSCI.17-24-09686.1997. ISSN 0270-6474, 1529-2401. PMID 9391023.
- ^ Schappelle (2017-08-11). "English: This image divides the insula into its anterior, mid, and posterior regions, with each being denoted by different colors." Wikimedia Commons.
- ^ a b c Polli, Frida E.; Barton, Jason J. S.; Cain, Matthew S.; Thakkar, Katharine N.; Rauch, Scott L.; Manoach, Dara S (2005-10-25). "Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission". Proceedings of the National Academy of Sciences of the United States of America. 102 (43): 15700–15705. doi:10.1073/pnas.0503657102. ISSN 0027-8424. PMC 1255733. PMID 16227444.
- ^ a b Etkin, Amit; Egner, Tobias; Kalisch, Raffael (2011-2). "Emotional processing in anterior cingulate and medial prefrontal cortex". Trends in cognitive sciences. 15 (2): 85–93. doi:10.1016/j.tics.2010.11.004. ISSN 1364-6613. PMC 3035157. PMID 21167765.
- ^ Hall, Geoff B (2011-09-08). "English: Sagittal MRI slice with highlighting indicating location of the anterior cingulate cortex." Wikimedia Commons.
- ^ Database Center for Life Science (2010-02-25). "English: Prefrontal cortex of left cerebral hemisphere. Shown in red." Wikimedia Commons.
- ^ a b c Grossmann, Tobias (2013). "The role of medial prefrontal cortex in early social cognition". Frontiers in Human Neuroscience. 7. doi:10.3389/fnhum.2013.00340. ISSN 1662-5161.
- ^ a b Euston, David R.; Gruber, Aaron J.; McNaughton, Bruce L (2012-12-20). "The Role of Medial Prefrontal Cortex in Memory and Decision Making". Neuron. 76 (6): 1057–1070. doi:10.1016/j.neuron.2012.12.002. ISSN 0896-6273. PMC 3562704. PMID 23259943.
- ^ Ortiz, Samantha; Latsko, Maeson S.; Fouty, Julia L.; Dutta, Sohini; Adkins, Jordan M.; Jasnow, Aaron M (2019-04-26). "Anterior cingulate cortex and ventral hippocampal inputs to the basolateral amygdala selectively control generalized fear". bioRxiv. 620237. doi:10.1101/620237.
- ^ a b Sridharan, Devarajan; Levitin, Daniel J.; Menon, Vinod (2008-08-26). "A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks". Proceedings of the National Academy of Sciences. 105 (34): 12569–12574. doi:10.1073/pnas.0800005105. ISSN 0027-8424, 1091-6490. PMID 18723676.
- ^ Evrard, Henry C.; Forro, Thomas; Logothetis, Nikos K (2012-05-10). "Von Economo Neurons in the Anterior Insula of the Macaque Monkey". Neuron. 74 (3): 482–489. doi:10.1016/j.neuron.2012.03.003. ISSN 0896-6273.
- ^ Hof, Patrick R.; Gucht, Estel Van Der (2007). "Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae (Cetacea, Mysticeti, Balaenopteridae)". The Anatomical Record. 290 (1): 1–31. doi:10.1002/ar.20407. ISSN 1932-8494.
- ^ a b Butti, Camilla; Sherwood, Chet C.; Hakeem, Atiya Y.; Allman, John M.; Hof, Patrick R (2009). "Total number and volume of Von Economo neurons in the cerebral cortex of cetaceans". Journal of Comparative Neurology. 515 (2): 243–259. doi:10.1002/cne.22055. ISSN 1096-9861.
- ^ Hakeem, Atiya Y.; Sherwood, Chet C.; Bonar, Christopher J.; Butti, Camilla; Hof, Patrick R.; Allman, John M (2009). "Von Economo Neurons in the Elephant Brain". The Anatomical Record. 292 (2): 242–248. doi:10.1002/ar.20829. ISSN 1932-8494.
- ^ Allman, John M.; Tetreault, Nicole A.; Hakeem, Atiya Y.; Park, Soyoung (2011). "The von economo neurons in apes and humans". American Journal of Human Biology. 23 (1): 5–21. doi:10.1002/ajhb.21136. ISSN 1520-6300.
- ^ a b c Gu, Xiaosi; Hof, Patrick R.; Friston, Karl J.; Fan, Jin (2013-10-15). "Anterior Insular Cortex and Emotional Awareness". The Journal of comparative neurology. 521 (15): 3371–3388. doi:10.1002/cne.23368. ISSN 0021-9967. PMC 3999437. PMID 23749500.
- ^ Allman, John; Hakeem, Atiya; Watson, Karli (2002-08-01). "Book Review: Two Phylogenetic Specializations in the Human Brain". The Neuroscientist. 8 (4): 335–346. doi:10.1177/107385840200800409. ISSN 1073-8584.
- ^ Menon, Vinod; Uddin, Lucina Q (2010-6). "Saliency, switching, attention and control: a network model of insula function". Brain structure & function. 214 (5-6): 655–667. doi:10.1007/s00429-010-0262-0. ISSN 1863-2653. PMC 2899886. PMID 20512370.
- ^ Mao, Can Van; Araujo, Mariana F. P.; Nishimaru, Hiroshi; Matsumoto, Jumpei; Tran, Ahn Hai; Hori, Etsuro; Ono, Taketoshi; Nishijo, Hisao (2017-02-01). "Pregenual Anterior Cingulate Gyrus Involvement in Spontaneous Social Interactions in Primates—Evidence from Behavioral, Pharmacological, Neuropsychiatric, and Neurophysiological Findings". Frontiers in Neuroscience. 11. doi:10.3389/fnins.2017.00034. ISSN 1662-4548. PMC 5285368. PMID 28203143.
- ^ Fajardo, C.; Escobar, M. I.; Buriticá, E.; Arteaga, G.; Umbarila, J.; Casanova, M. F.; Pimienta, H (2008-04-25). "Von Economo neurons are present in the dorsolateral (dysgranular) prefrontal cortex of humans". Neuroscience Letters. 435 (3): 215–218. doi:10.1016/j.neulet.2008.02.048. ISSN 0304-3940.
- ^ Cauda, F; Geminiani, GC; Vercelli, A (2014-03-14). "English: Areas characterized by the presence of von Economo’s neurons (VENs) in the human brain. Red: the anterior insula, Pink: Brodmann area 9 frontal cortex, Brown: Brodmann area 24 anterior cingulate cortex. Different intensities in the colors stand for different densities of VENs, which are more frequent on the right than on the left side in area 9 and in the anterior insula, and in cingulate area 24a > 24b > 24c." Wikimedia Commons.
- ^ Nimchinsky, Esther A.; Vogt, Brent A.; Morrison, John H.; Hof, Patrick R (1995). "Spindle neurons of the human anterior cingul. Ate cortex". Journal of Comparative Neurology. 355 (1): 27–37. doi:10.1002/cne.903550106. ISSN 1096-9861.
- ^ Ozier, Douglas; Westbury, Chris (2013-05-07). "English: Von Economo neurons. As illustrated with the halftone gradient in this figure, which is labeled with Brodmann’s area numbers, the main concentration of Von Economo neurons in the human brain is found in BA24, with a decreasing density moving dorsally." Wikimedia Commons.
- ^ Cobos, Inma; Seeley, William W (2015-01-01). "Human von Economo Neurons Express Transcription Factors Associated with Layer V Subcerebral Projection Neurons". Cerebral Cortex. 25 (1): 213–220. doi:10.1093/cercor/bht219. ISSN 1047-3211.
- ^ Hodge, Rebecca D.; Miller, Jeremy A.; Novotny, Mark; Kalmbach, Brian E.; Ting, Jonathan T.; Bakken, Trygve E.; Aevermann, Brian D.; Barkan, Eliza R.; Berkowitz-Cerasano, Madeline L.; Cobbs, Charles; Diez-Fuertes, Francisco; Ding, Song-Lin; McCorrison, Jamison; Schork, Nicholas J.; Shehata, Soraya I.; Smith, Kimberly A.; Sunkin, Susan M.; Tran, Danny N.; Venepally, Pratap; Yanny, Anna Marie; Steemers, Frank J.; Phillips, John W.; Bernard, Amy; Koch, Christof; Lasken, Roger S.; Scheuermann, Richard H.; Lein, Ed S (2020-03-03). "Transcriptomic evidence that von Economo neurons are regionally specialized extratelencephalic-projecting excitatory neurons". Nature Communications. 11 (1): 1–14. doi:10.1038/s41467-020-14952-3. ISSN 2041-1723.
- ^ Seeley, William W (2008-12). "Selective functional, regional, and neuronal vulnerability in frontotemporal dementia". Current Opinion in Neurology. 21 (6): 701–707. doi:10.1097/WCO.0b013e3283168e2d. ISSN 1350-7540.
- ^ Allman, John M.; Watson, Karli K.; Tetreault, Nicole A.; Hakeem, Atiya Y (2005-08-01). "Intuition and autism: a possible role for Von Economo neurons". Trends in Cognitive Sciences. 9 (8): 367–373. doi:10.1016/j.tics.2005.06.008. ISSN 1364-6613, 1879-307X. PMID 16002323.
- ^ Qadir, Houman; Krimmel, Samuel R.; Mu, Chaoqi; Poulopoulos, Alexandros; Seminowicz, David A.; Mathur, Brian N (2018). "Structural Connectivity of the Anterior Cingulate Cortex, Claustrum, and the Anterior Insula of the Mouse". Frontiers in Neuroanatomy. 12. doi:10.3389/fnana.2018.00100. ISSN 1662-5129.
- ^ a b c Menon, Vinod (2011-10-01). "Large-scale brain networks and psychopathology: a unifying triple network model". Trends in Cognitive Sciences. 15 (10): 483–506. doi:10.1016/j.tics.2011.08.003. ISSN 1364-6613, 1879-307X. PMID 21908230.
- ^ Sridharan, Devarajan; Levitin, Daniel J.; Menon, Vinod (2008-08-26). "A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks". Proceedings of the National Academy of Sciences of the United States of America. 105 (34): 12569–12574. doi:10.1073/pnas.0800005105. ISSN 0027-8424. PMC 2527952. PMID 18723676.
- ^ Nekovarova, Tereza; Fajnerova, Iveta; Horacek, Jiri; Spaniel, Filip (2014). "Bridging disparate symptoms of schizophrenia: a triple network dysfunction theory". Frontiers in Behavioral Neuroscience. 8. doi:10.3389/fnbeh.2014.00171. ISSN 1662-5153.
- ^ Li, Wanqing; Mai, Xiaoqin; Liu, Chao (2014-02-24). "The default mode network and social understanding of others: what do brain connectivity studies tell us". Frontiers in Human Neuroscience. 8. doi:10.3389/fnhum.2014.00074. ISSN 1662-5161. PMC 3932552. PMID 24605094.
- ^ Andrews-Hanna, Jessica R.; Smallwood, Jonathan; Spreng, R. Nathan (2014-5). "The default network and self-generated thought: component processes, dynamic control, and clinical relevance". Annals of the New York Academy of Sciences. 1316 (1): 29–52. doi:10.1111/nyas.12360. ISSN 0077-8923. PMC 4039623. PMID 24502540.
- ^ Mars, Rogier B.; Neubert, Franz-Xaver; Noonan, MaryAnn P.; Sallet, Jerome; Toni, Ivan; Rushworth, Matthew F. S (2012). "On the relationship between the “default mode network” and the “social brain”". Frontiers in Human Neuroscience. 6. doi:10.3389/fnhum.2012.00189. ISSN 1662-5161.
- ^ Wheeler, M.; Smelser, Neil J.; Baltes, Paul B (2001-01-01). "Episodic and Autobiographical Memory: Psychological and Neural Aspects". 4714–4717.
- ^ a b Allman, John M.; Tetreault, Nicole A.; Hakeem, Atiya Y.; Manaye, Kebreten F.; Semendeferi, Katerina; Erwin, Joseph M.; Park, Soyoung; Goubert, Virginie; Hof, Patrick R (2011-4). "The von Economo neurons in fronto-insular and anterior cingulate cortex". Annals of the New York Academy of Sciences. 1225: 59–71. doi:10.1111/j.1749-6632.2011.06011.x. ISSN 0077-8923. PMC 3140770. PMID 21534993.