Given that the social mindset is facilitated by the association of brainstem-response signals from the anterior insula with episodic memories in the medial prefrontal cortex, the lack of the social mindset involves a significant relative deficiency in the formation of these associations in comparison to the formation of episodic memories that regulate innate survival mechanisms.
In our case, this appears to be facilitated by an overdeveloped cerebral cortex in combination with a comparatively underdeveloped and underlateralised anterior insula and anterior cingulate cortex (ACC). On this page, this is shown on MRIs of myself and in evidence from published literature on our diagnoses and symptoms.
Although anterior-insula and ventral-ACC function is reduced in our condition, this dynamic appears to manifest with hyperactivity of the dorsal ACC, as represented by our obsessive–compulsive disorder–like symptoms and my tic disorder, and also appears to manifest with hyperactivity of the posterior insula, the region linked to our sensory hypersensitivities.
Anterior insular cortex
Studies have generally found reduced activity and hypoconnectivity of the anterior insula (especially the right anterior insula) in autism spectrum disorder (ASD).
Damage to the insula has also been recorded to result in sensory hypersensitivity, especially manifesting as disgust, as well as reduced reward from smoking and money.
Autism spectrum disorder
- A 2009 meta-analysis found consistent hypoactivity in the right insula during social tasks in those with ASD.[1]
- A 2009 study found that reduced functional connectivity between the anterior mid-insula and pregenual anterior cingulate cortex correlated with autistic traits.[2]
- A 2016 study found a lack of an anterior insular region responsive to emotional processes in a sample of those with ASD.[3]
- A 2012 study found reduced resting-state connectivity between the right anterior insula and the nodes of the default mode network in those with ASD.[4]
Sensory issues
- A 1965 study reported a man with a diffuse insular stroke suffering from a strong aversion to bright lights, smells (including formerly pleasant cooking odours), sounds and tactile sensation.[5]
- Left posterior insular stroke and right insular resection for epilepsy has resulted in increased sensitivity to odours and taste, especially manifesting as displeasure.[6]
On the converse, other diffuse insular strokes have resulted in hyposensitivity, including to taste, touch, pain, temperature and disgust, as well as other features such as heart-rate abnormalities, motor-related balance problems and hemispatial neglect.[7]
A 2013 review discussed the sensory and social roles of the insula with regards to ASD and reviewed the hypothesis that dysfunction of the anterior-insula–anterior-cingulate-cortex network was the core pathology of ASD.[8]
Reward-/aversion-related features
- A 2015 study[9] found that those with insular stroke could lack the gambler’s fallacy and near-miss effect, in which a string of losses or a near miss to success compels one to continue gambling.[6]
- Strokes affecting the insula in smokers have been found to result in an ability to quit smoking ‘easily, immediately, without relapse and without persistence of the urge to smoke’.[10][11]
Myself
I was given an MRI at age 14. I later obtained the MRI via a freedom-of-information request.
Due to the significant involvement of the anterior insula in the social mindset, I have provided slices of my anterior insula from my MRI at age 14. I have compared the MRI to the closest available male reference MRI in age (20) on Radiopaedia for which clear axial and coronal views were available.
Compared to this MRI, there is a slight reduction in the right anterior insula compared to the left and a general reduction in both anterior insulae compared to the rest of the cortex (such as the prefrontal cortex). The rest of the cortex has grown out further beyond the insulae.
On each MRI, ‘R’ and ‘L’ refer to the right and left hemispheres of the brain, respectively. None of the following MRIs are to scale.

Source: [13]. Licence: CC-BY-NC-SA-3.0.
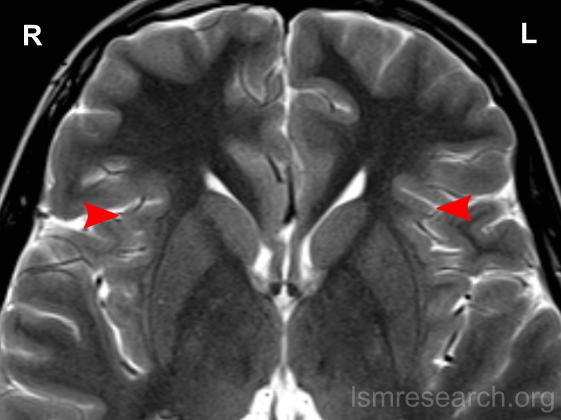
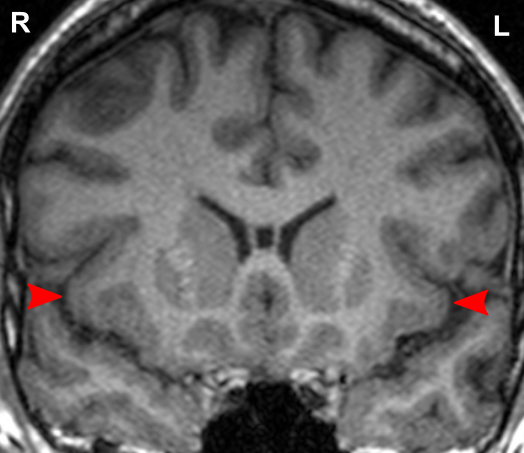
Source: [13]. Licence: CC-BY-NC-SA-3.0.
Anterior cingulate cortex
Studies have generally found deficits in the ventral anterior cingulate cortex (ACC) in ASD and hyperactivity of the dorsal ACC in obsessive–compulsive disorder (OCD).
Autism spectrum disorder
- The same 2009 meta-analysis that found consistent hypoactivity in the right insula during social tasks in those with autism spectrum disorder also found consistent hypoactivity in the ventral ACC (but not dorsal ACC) during these tasks.[1]
- As mentioned in the previous section, a 2009 study found that reduced functional connectivity between the anterior mid-insula and pregenual ACC correlated with autistic traits.[2]
Obsessive–compulsive disorder
- A 2017 review found that the dorsal ACC was generally hyperactive in the resting state in OCD.[14]
- A 2010 study found that the dorsal ACC and ventromedial prefrontal cortex (vmPFC) were hyperactive and had increased connectivity in a number identification task in those with OCD. At rest, however, the dorsal ACC had decreased connectivity with the right anterior insula, vmPFC and posterior cingulate cortex.[15]
Anterior cingulotomies (cutting of the ACC white matter) used to be used to treat OCD and anxiety disorders. Disruption of the anterior cingulum bundle results in a happy demeanour without learnt anxieties.[16][17] It also reduces aversion to pain and can precipitate self-mutilation in rats.[16]
Myself
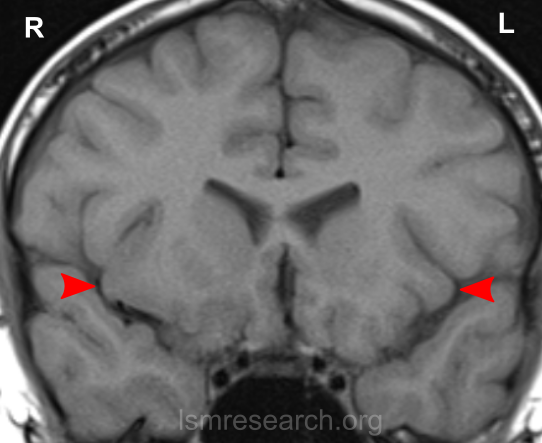
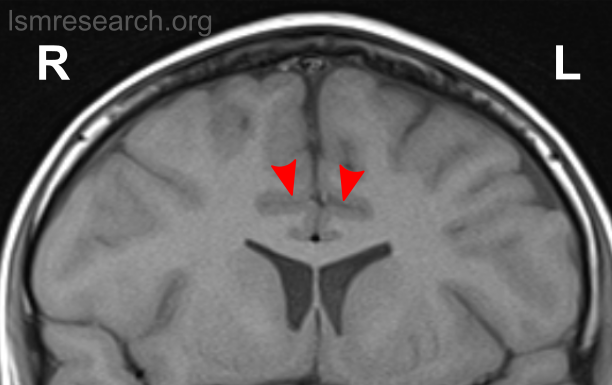
Due to the significant involvement of the rostral/ventral/pregenual ACC in the social mindset, I have provided slices of my ACC from my MRI at age 14.
Compared to the closest available male reference MRI in age (20) on Radiopaedia for which clear sagittal, axial and coronal views were available, both rostral ACCs show a size reduction relative to the rest of the cortex that is more prominent in the right hemisphere than the left.
None of the following MRIs are to scale.
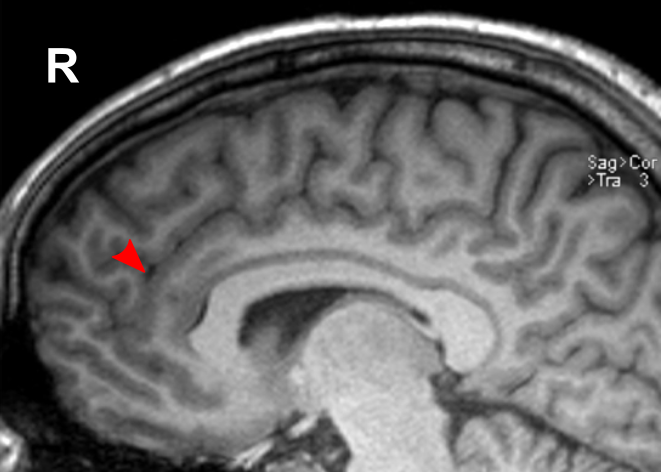
Source: [13]. Licence: CC-BY-NC-SA-3.0.
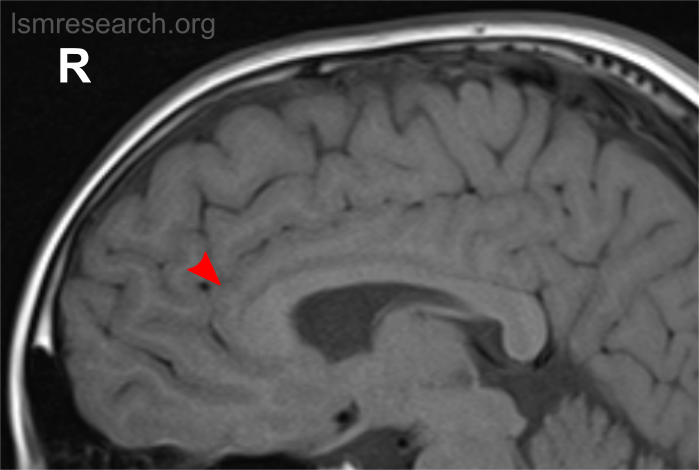
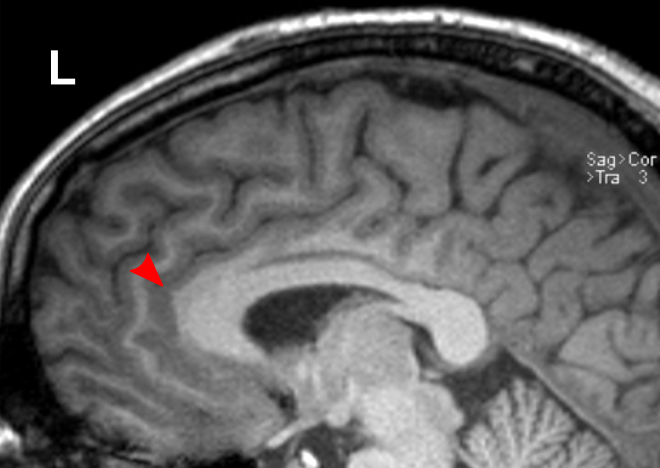
Source: [13]. Licence: CC-BY-NC-SA-3.0.

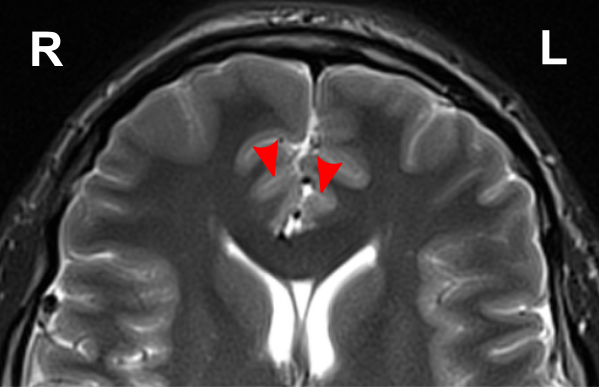
Source: [13]. Licence: CC-BY-NC-SA-3.0.
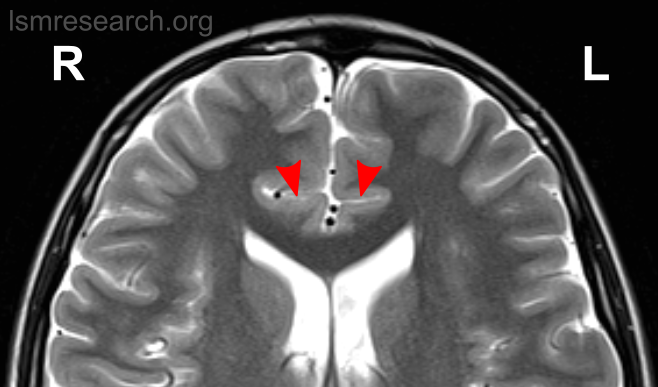
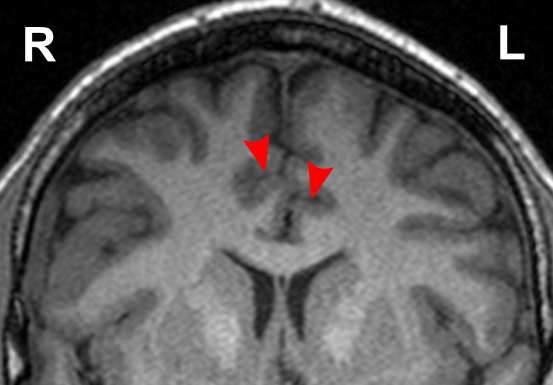
Source: [13]. Licence: CC-BY-NC-SA-3.0.
Megalencephaly
Autism spectrum disorder
Megalencephaly or macrocephaly (brain size or head size at or above the 98th percentile) is found in autism spectrum disorder at rates of around 20%.[18]
Brain growth in these cases has been established to dramatically increase within the first year of life before plateauing closer to average prior to adulthood. However, reported neonatal and adult brain sizes vary.[19][20]
Also not consistent is the reported association between brain size in autism and symptom presentation (less severe core symptoms,[21][22] more severe social deficits,[20][23][24] more low-functioning autism,[25] more high-functioning autism/Asperger syndrome[26]), region-specific growth (prefrontal;[27] only temporal, parietal and occipital[19]) and intelligence (low, average or high despite a common genetic basis with intelligence genes[28]).
Megalencephaly in autism is largely restricted to males. A 1996 study of 35 individuals with autism found significantly increased brain volume in the males but not the females,[19] and a 1995 study of a sample of 148 children with autism found that megalencephaly in autism was found overwhelmingly in males; whilst the male-to-female ratio was 5:1 for the entire sample, this rose to 27:1 for those with megalencephaly.[24]
Ourselves
My and my friend’s brains both followed the above-described widely reported pattern of growth, rapidly increasing in early childhood before coming closer to average prior to adulthood.
I was born with a head circumference of around 34.5 cm (~25th–50th percentile). By age 1, this had increased to 49 cm (~90th–99th percentile).
The following images show each of us in early childhood.
The difference can also be seen at the age of 14 in myself, shown in the following to-scale comparison with a reference axial MRI of a 15-year-old male from Radiopaedia.
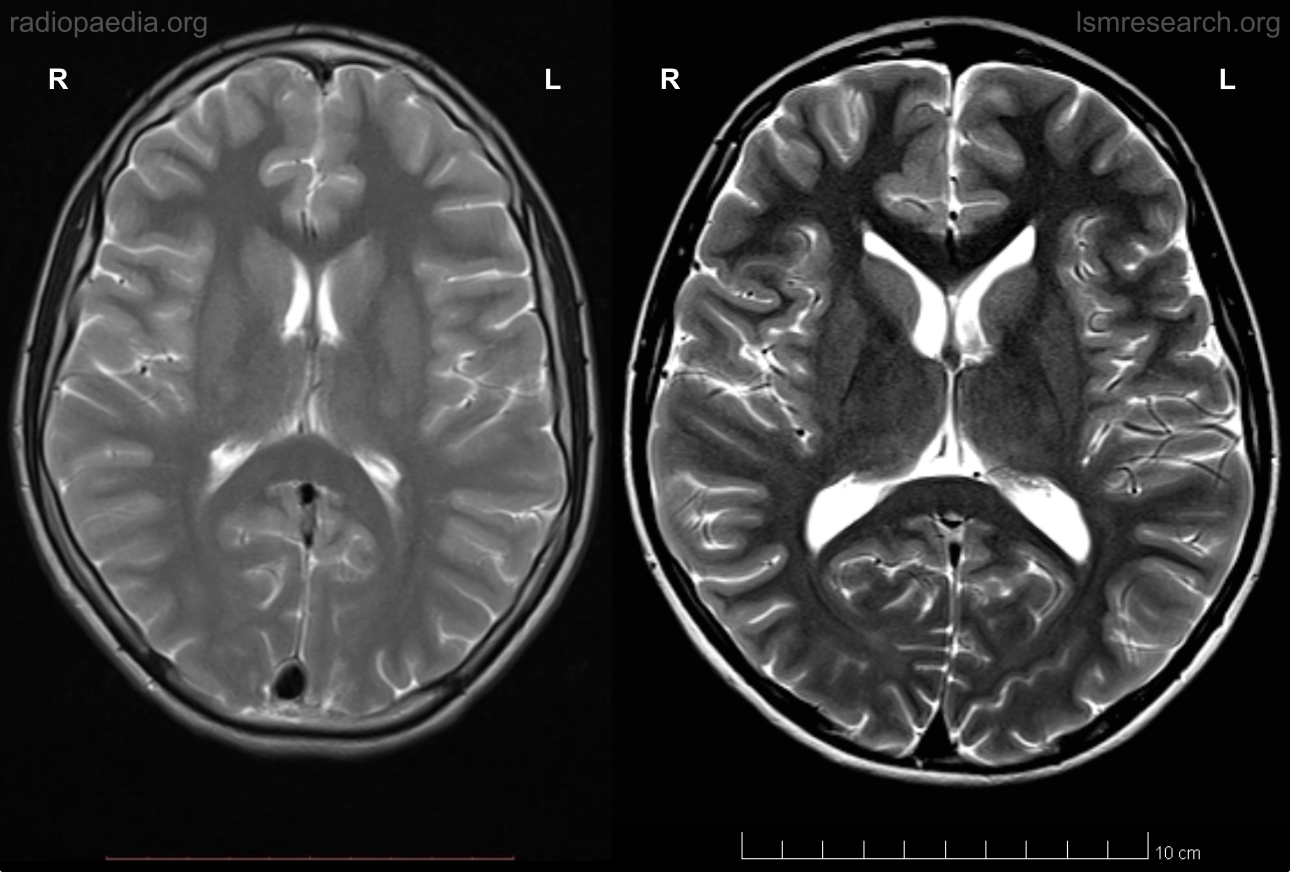
Source: [29]. Licence: CC-BY-NC-SA-3.0.
Despite the inconsistency in reported correlations to factors in autism, there appears to be a correlation to intelligence in our cases, which is described on Other features of our lack of the social mindset.
My MRI also shows a mild degree of ventricular enlargement. Ventriculomegaly at birth, where not due to cerebrospinal-fluid obstruction, has been associated with increased cortical grey matter (without white-matter increase) and has been found in overgrowth disorders (such as Sotos syndrome, which is associated with autistic traits[30]) and high-functioning autism.[31]
A 2014 study proposed that in these cases, surface area of the ventricles may be increased due to an initially increased neuronal-progenitor-cell population in the ventricular and subventricular zones.[31]
Diagram
These differences lend to a diagram that can be used to roughly illustrate the neurology of a typical brain with the social mindset in comparison to the neurology of our brains with our lack of the social mindset.
In the following diagram, the blue section is the anterior insular cortex and anterior cingulate cortex (the social-mindset brain regions), while the red section is the rest of the frontal and temporal lobes.
In the coronal MRI slices, which are not to scale, the ratio of the area of the social-mindset brain regions to the rest of the frontal and temporal lobes in the reference 20-year-old male is around 1:7.6. In myself at age 14, it is around 1:10.6, around 39% smaller.
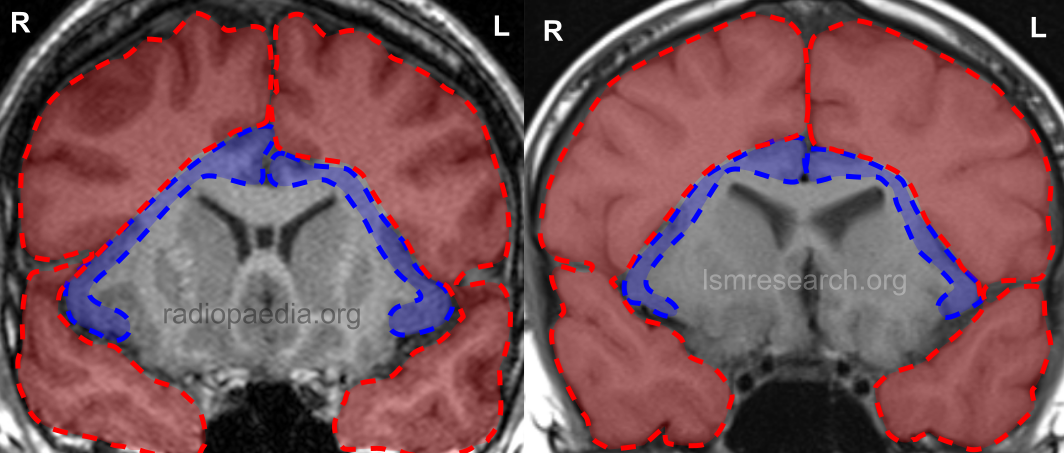
Source: [13]. Licence: CC-BY-NC-SA-3.0.
References
- ^ a b Di Martino, Adriana; Ross, Kathryn; Uddin, Lucina Q.; Sklar, Andrew B.; Castellanos, F. Xavier; Milham, Michael P (2009-01-01). "Functional Brain Correlates of Social and Non-Social Processes in Autism Spectrum Disorders: an ALE Meta-Analysis". Biological psychiatry. 65 (1): 63–74. doi:10.1016/j.biopsych.2008.09.022. ISSN 0006-3223. PMC 2993772. PMID 18996505.
- ^ a b Di Martino, Adriana; Shehzad, Zarrar; Kelly, Clare; Roy, Amy Krain; Gee, Dylan G.; Uddin, Lucina Q.; Gotimer, Kristin; Klein, Donald F.; Castellanos, F. Xavier; Milham, Michael P (2009-08). "Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults". The American Journal of Psychiatry. 166 (8): 891–899. doi:10.1176/appi.ajp.2009.08121894. ISSN 1535-7228. PMC 3075727. PMID 19605539.
- ^ Yamada, Takashi; Itahashi, Takashi; Nakamura, Motoaki; Watanabe, Hiromi; Kuroda, Miho; Ohta, Haruhisa; Kanai, Chieko; Kato, Nobumasa; Hashimoto, Ryu-ichiro (2016-10-05). "Altered functional organization within the insular cortex in adult males with high-functioning autism spectrum disorder: evidence from connectivity-based parcellation". Molecular Autism. 7 (1): 41. doi:10.1186/s13229-016-0106-8. ISSN 2040-2392.
- ^ Guo, Xiaonan; Duan, Xujun; Suckling, John; Chen, Heng; Liao, Wei; Cui, Qian; Chen, Huafu (2019). "Partially impaired functional connectivity states between right anterior insula and default mode network in autism spectrum disorder". Human Brain Mapping. 40 (4): 1264–1275. doi:10.1002/hbm.24447. ISSN 1097-0193.
- ^ Spreen, Otfried; Benton, Arthur L.; Fincham, Richard W (1965-07-01). "Auditory Agnosia Without Aphasia". Archives of Neurology. 13 (1): 84–92. doi:10.1001/archneur.1965.00470010088012. ISSN 0003-9942.
- ^ a b Uddin, Lucina Q.; Nomi, Jason S.; Hebert-Seropian, Benjamin; Ghaziri, Jimmy; Boucher, Olivier (2017-7). "Structure and function of the human insula". Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 34 (4): 300–306. doi:10.1097/WNP.0000000000000377. ISSN 0736-0258. PMC 6032992. PMID 28644199.
- ^ Ibañez, Agustin; Gleichgerrcht, Ezequiel; Manes, Facundo (2010-06-01). "Clinical effects of insular damage in humans". Brain Structure and Function. 214 (5): 397–410. doi:10.1007/s00429-010-0256-y. ISSN 1863-2661.
- ^ Toyomaki, Atsuhito; Murohashi, Harumitsu (2013). "“Salience network” dysfunction hypothesis in autism spectrum disorders". Japanese Psychological Research. 55 (2): 175–185. doi:10.1111/jpr.12012. ISSN 1468-5884.
- ^ Yeo, B. T. Thomas; Krienen, Fenna M.; Eickhoff, Simon B.; Yaakub, Siti N.; Fox, Peter T.; Buckner, Randy L.; Asplund, Christopher L.; Chee, Michael W.L (2015-10). "Functional Specialization and Flexibility in Human Association Cortex". Cerebral Cortex (New York, NY). PubMed Central. 25 (10): 3654–3672. doi:10.1093/cercor/bhu217. ISSN 1047-3211. PMC 4598819. PMID 25249407.
- ^ Naqvi, Nasir H.; Rudrauf, David; Damasio, Hanna; Bechara, Antoine (2007-01-26). "Damage to the insula disrupts addiction to cigarette smoking". Science (New York, N.Y.). 315 (5811): 531–534. doi:10.1126/science.1135926. ISSN 1095-9203. PMC 3698854. PMID 17255515.
- ^ Suñer-Soler, Rosa; Grau, Armando; Eugenia Gras, Maria; Font-Mayolas, Sílvia; Silva, Yolanda; Dávalos, Antonio; Cruz, Verónica; Rodrigo, Joana; Serena, Joaquín (2012-01-01). "Smoking Cessation 1 Year Poststroke and Damage to the Insular Cortex". Stroke. ahajournals.org (Atypon). 43 (1): 131–136. doi:10.1161/STROKEAHA.111.630004.
- ^ Gaznick, Natassia; Tranel, Daniel; McNutt, Ashton; Bechara, Antoine (2014-4). "Basal Ganglia Plus Insula Damage Yields Stronger Disruption of Smoking Addiction Than Basal Ganglia Damage Alone". Nicotine & Tobacco Research. PubMed Central. 16 (4): 445–453. doi:10.1093/ntr/ntt172. ISSN 1462-2203. PMC 3954424. PMID 24169814.
- ^ a b c d e f g Case contributed by Assoc Prof Frank Gaillard. "Normal brain MRI (non-focal epilepsy protocol) | Radiology Case | Radiopaedia.org". Radiopaedia.
- ^ McGovern, Robert A.; Sheth, Sameer A (2017-01-01). "Role of the dorsal anterior cingulate cortex in obsessive-compulsive disorder: converging evidence from cognitive neuroscience and psychiatric neurosurgery". Journal of Neurosurgery. 126 (1): 132–147. doi:10.3171/2016.1.JNS15601. ISSN 1933-0693, 0022-3085.
- ^ Fitzgerald, Kate Dimond; Stern, Emily R.; Angstadt, Mike; Nicholson-Muth, Karen; Maynor, McKenzie; Welsh, Robert C.; Hanna, Gregory L.; Taylor, Stephan F (2010-12-01). "Altered function and connectivity of the medial frontal cortex in pediatric obsessive compulsive disorder". Biological psychiatry. 68 (11): 1039–1047. doi:10.1016/j.biopsych.2010.08.018. ISSN 0006-3223. PMC 2988474. PMID 20947065.
- ^ a b Bubb, Emma J.; Metzler-Baddeley, Claudia; Aggleton, John P (2018-09-01). "The cingulum bundle: Anatomy, function, and dysfunction". Neuroscience & Biobehavioral Reviews. 92: 104–127. doi:10.1016/j.neubiorev.2018.05.008. ISSN 0149-7634.
- ^ "Cingulum stimulation enhances positive affect and anxiolysis to facilitate awake craniotomy". The Journal of Clinical Investigation. 129 (3): 1152–1166. doi:10.1172/JCI120110. ISSN 0021-9738. PMC 6391096. PMID 30589643.
- ^ Lainhart, Janet E.; Lange, Nicholas (2011-11-09). "Increased Neuron Number and Head Size in Autism". JAMA. 306 (18): 2031–2032. doi:10.1001/jama.2011.1633. ISSN 0098-7484.
- ^ a b c Piven, J.; Arndt, S.; Bailey, J.; Andreasen, N (1996-04). "Regional brain enlargement in autism: a magnetic resonance imaging study". Journal of the American Academy of Child and Adolescent Psychiatry. 35 (4): 530–536. doi:10.1097/00004583-199604000-00020. ISSN 0890-8567. PMID 8919716.
- ^ a b Libero, Lauren E.; Nordahl, Christine W.; Li, Deana D.; Ferrer, Emilio; Rogers, Sally J.; Amaral, David G (2016). "Persistence of megalencephaly in a subgroup of young boys with autism spectrum disorder". Autism Research. 9 (11): 1169–1182. doi:10.1002/aur.1643. ISSN 1939-3806.
- ^ Lainhart, Janet E.; Piven, Joseph; Wzorek, Maryann; Landa, Rebecca; Santangelo, Susan L.; Coon, Hilary; Folstein, Susan E (1997-02-01). "Macrocephaly in Children and Adults With Autism". Journal of the American Academy of Child & Adolescent Psychiatry. 36 (2): 282–290. doi:10.1097/00004583-199702000-00019. ISSN 0890-8567.
- ^ Dementieva, Yulia A.; Vance, Danica D.; Donnelly, Shannon L.; Elston, Leigh A.; Wolpert, Chantelle M.; Ravan, Sarah A.; DeLong, G. Robert; Abramson, Ruth K.; Wright, Harry H.; Cuccaro, Michael L (2005-02-01). "Accelerated head growth in early development of individuals with autism". Pediatric Neurology. 32 (2): 102–108. doi:10.1016/j.pediatrneurol.2004.08.005. ISSN 0887-8994.
- ^ Hazlett, Heather Cody; Gu, Hongbin; Munsell, Brent C.; Kim, Sun Hyung; Styner, Martin; Wolff, Jason J.; Elison, Jed T.; Swanson, Meghan R.; Zhu, Hongtu; Botteron, Kelly N.; Collins, D. Louis; Constantino, John N.; Dager, Stephen R.; Estes, Annette M.; Evans, Alan C.; Fonov, Vladimir S.; Gerig, Guido; Kostopoulos, Penelope; McKinstry, Robert C.; Pandey, Juhi; Paterson, Sarah; Pruett, John R.; Schultz, Robert T.; Shaw, Dennis W.; Zwaigenbaum, Lonnie; Piven, Joseph (2017-02-15). "Early brain development in infants at high risk for autism spectrum disorder". Nature. PubMed. 542 (7641): 348–351. doi:10.1038/nature21369. ISSN 1476-4687. PMC 5336143. PMID 28202961.
- ^ a b Davidovitch, Michael; Patterson, Bonnie; Gartside, Peter (2016-07-02). "Head Circumference Measurements in Children With Autism:". Journal of Child Neurology. journals.sagepub.com. doi:10.1177/088307389601100509.
- ^ Sacco, Roberto; Gabriele, Stefano; Persico, Antonio M (2015-11-30). "Head circumference and brain size in autism spectrum disorder: A systematic review and meta-analysis". Psychiatry Research: Neuroimaging. 234 (2): 239–251. doi:10.1016/j.pscychresns.2015.08.016. ISSN 0925-4927.
- ^ Gillberg, Christopher; Souza, Linda De (2002). "Head circumference in autism, Asperger syndrome, and ADHD: a comparative study". Developmental Medicine & Child Neurology. 44 (5): 296–300. doi:10.1111/j.1469-8749.2002.tb00814.x. ISSN 1469-8749.
- ^ Courchesne, Eric; Mouton, Peter R.; Calhoun, Michael E.; Semendeferi, Katerina; Ahrens-Barbeau, Clelia; Hallet, Melodie J.; Barnes, Cynthia Carter; Pierce, Karen (2011-11-09). "Neuron number and size in prefrontal cortex of children with autism". JAMA. 306 (18): 2001–2010. doi:10.1001/jama.2011.1638. ISSN 1538-3598. PMID 22068992.
- ^ Crespi, Bernard J (2016-06-30). "Autism As a Disorder of High Intelligence". Frontiers in Neuroscience. 10. doi:10.3389/fnins.2016.00300. ISSN 1662-4548. PMC 4927579. PMID 27445671.
- ^ Case contributed by Dr Hidayatullah Hamidi. "Normal head MR angiogram | Radiology Case | Radiopaedia.org". Radiopaedia.
- ^ Lane, Chloe; Milne, Elizabeth; Freeth, Megan (2017). "Characteristics of Autism Spectrum Disorder in Sotos Syndrome". Journal of Autism and Developmental Disorders. 47 (1): 135–143. doi:10.1007/s10803-016-2941-z. ISSN 0162-3257. PMC 5222916. PMID 27771801.
- ^ a b Kyriakopoulou, Vanessa; Vatansever, Deniz; Elkommos, Samia; Dawson, Sarah; McGuinness, Amy; Allsop, Joanna; Molnár, Zoltán; Hajnal, Joseph; Rutherford, Mary (2014-08-01). "Cortical Overgrowth in Fetuses With Isolated Ventriculomegaly". Cerebral Cortex. 24 (8): 2141–2150. doi:10.1093/cercor/bht062. ISSN 1047-3211.
- ^ a b Di Martino, Adriana; Shehzad, Zarrar; Kelly, Clare; Roy, Amy Krain; Gee, Dylan G.; Uddin, Lucina Q.; Gotimer, Kristin; Klein, Donald F.; Castellanos, F. Xavier; Milham, Michael P (2009-08). "Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults". The American Journal of Psychiatry. 166 (8): 891–899. doi:10.1176/appi.ajp.2009.08121894. ISSN 1535-7228. PMC 3075727. PMID 19605539.

